Pharma MES Platform
Pharma MES Platform

Vimachem’s Pharma MES Platform is a modular, AI-driven system built specifically for pharmaceutical and biotech companies. It streamlines production, ensures regulatory compliance, and enables real-time monitoring of processes and equipment. With advanced analytics, paperless operations, and machine integration, it empowers manufacturers to transform digitally with confidence. Whether you're digitizing batch records or optimizing warehouse traceability, Vimachem adapts to your workflows and scales as you grow.
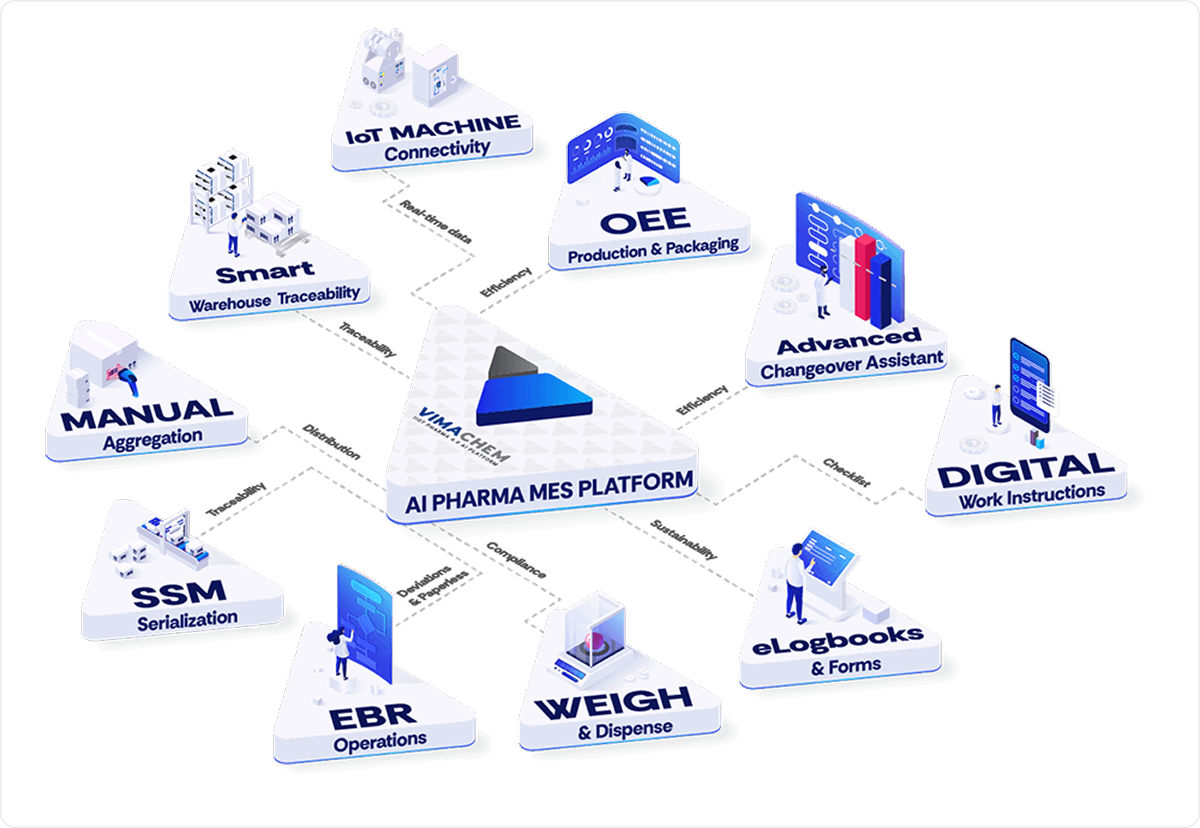
Automates batch documentation, ensures GMP compliance, and provides full traceability—eliminating paper-based errors and accelerating production review.
Improves accuracy and efficiency during raw material handling with guided workflows and real-time checks, reducing manual entry errors.
Connects shop floor machines to the MES, enabling real-time performance tracking, predictive maintenance, and centralized data visibility.
Tracks and manages serialization and aggregation, ensuring compliance with DSCSA, EU FMD, and other global traceability regulations.
Monitors materials across storage and production zones, ensuring lot-wise tracking, FIFO enforcement, and seamless inventory reconciliation.